Physics and Mathematics
Excess Pressure Inside a Liquid Drop
1. Statement of the Concept
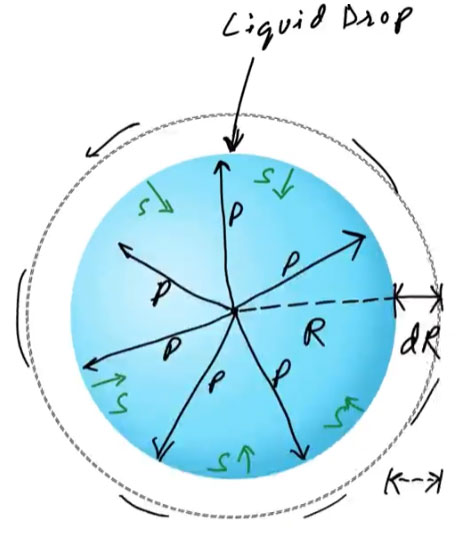
When a liquid drop is formed, surface tension acts along its surface, trying to minimize the surface area. This tension creates a difference in pressure between the inside and outside of the drop — the pressure inside the drop becomes greater than the pressure outside.
This pressure difference is called Excess Pressure.
2. Explanation and Mathematical Derivation
Consider a spherical liquid drop of radius [r] and surface tension [T].
- Let the internal pressure be greater than the external pressure by [p].
- Due to this excess pressure, if the radius of the drop increases by a small amount [dr], then:
- Work done by excess pressure = [p × 4πr² × dr]
- Increase in surface energy = [T] [× \text{Increase in surface area}] = [T × (8πr × dr)]
At equilibrium,
[ \text{Work done by pressure}] [= \text{Increase in surface energy} ]
So,
[ p × 4πr² × dr = T × 8πr × dr ]
Simplifying,
[ p = \dfrac{2T}{r} ]
Hence,
Excess pressure inside a liquid drop: [\boxed{p = \dfrac{2T}{r}}]
3. Dimensions and Units
- Formula: [p = \dfrac{2T}{r}]
- Dimensions of T: [M¹L⁰T⁻²]
- Dimensions of p: [ML⁻¹T⁻²]
- SI Unit: Pascal (Pa)
- CGS Unit: dyne/cm²
4. Key Features
- Excess pressure arises due to surface tension acting to contract the surface.
- Smaller the radius, greater the excess pressure.
- For soap bubbles, both inner and outer surfaces contribute, hence
[ p = \dfrac{4T}{r} ] - Explains why small droplets tend to merge into larger drops (to reduce energy).
5. Important Formulas to Remember
| Case | Excess Pressure (p) | Remarks |
|---|---|---|
| Liquid Drop | [p = \dfrac{2T}{r}] | Single surface |
| Soap Bubble | [p = \dfrac{4T}{r}] | Two surfaces |
| Liquid Jet (Cylinder) | [p = \dfrac{T}{r}] | Curvature in one direction |
6. Conceptual Questions with Solutions
1. Why does excess pressure arise inside a liquid drop?
Because the surface tension acts inward, reducing surface area and requiring higher internal pressure to balance it.
2. Why is excess pressure inversely proportional to radius?
Since [p = \dfrac{2T}{r}], smaller radius means larger curvature and thus higher pressure difference.
3. What happens to excess pressure when the drop radius doubles?
It becomes half, as [p ∝ \dfrac{1}{r}].
4. Why is excess pressure more in a soap bubble than in a liquid drop?
A soap bubble has two surfaces, inner and outer, both contributing to surface tension, giving [p = \dfrac{4T}{r}].
5. What is the significance of surface tension in excess pressure?
Surface tension creates the pressure difference by pulling the surface inward.
6. How does excess pressure explain the merging of small drops?
Small drops have high internal pressure, so they merge into larger ones to lower total energy.
7. Does excess pressure depend on the liquid’s density?
No, it depends only on surface tension [T] and radius [r].
8. What will happen if the surface tension of a liquid increases?
The excess pressure increases, as [p ∝ T].
9. Why is it difficult to form small droplets?
Because smaller droplets require greater internal pressure to balance surface tension.
10. How does temperature affect excess pressure?
As temperature increases, surface tension decreases, thus reducing excess pressure.
11. Why does a balloon burst when inflated too much?
The pressure difference exceeds the elastic limit of the surface film.
12. What is the excess pressure if surface tension is zero?
Then [p = 0]; the concept of excess pressure vanishes.
13. How does excess pressure affect boiling point in small bubbles?
Higher internal pressure raises the boiling point locally.
14. How does excess pressure vary for different liquids?
Liquids with higher surface tension have higher excess pressure.
15. Can we neglect excess pressure in large drops?
Yes, since [r] is large, [p] becomes very small.
7. FAQ / Common Misconceptions
1. Is excess pressure caused by gravity?
No, it is purely due to surface tension, not gravitational forces.
2. Does excess pressure act tangentially?
No, it acts **normally inward** to the surface.
3. Is the excess pressure the same for all drops?
No, it depends on radius — smaller drops have more excess pressure.
4. Why does a soap bubble have double the excess pressure?
Because it has two surfaces (inner and outer) experiencing surface tension.
5. Does surface tension depend on shape?
No, only on the nature of liquid and temperature.
6. Can excess pressure exist without surface tension?
No, without surface tension, there is no restoring force to create pressure difference.
7. Does external air pressure affect excess pressure formula?
No, the formula [p = \dfrac{2T}{r}] already measures the difference.
8. Why is the pressure higher inside the drop?
To balance the inward pull of surface tension maintaining equilibrium.
9. Is excess pressure measurable directly?
Indirectly, using experiments based on capillarity or bubble formation.
10. What happens when the drop radius tends to infinity?
The surface becomes flat, and excess pressure approaches zero.
8. Practice Questions (with Step-by-Step Solutions)
Q1. Calculate the excess pressure inside a water drop of radius [0.0015 cm]. Surface tension of water is [T = 7.2 × 10⁻² N/m].
Solution:
[p] [= \dfrac{2T}{r}] [= \dfrac{2 × 7.2 × 10^{-2}}{1.5 × 10^{-5}}] [= 9.6 × 10^3 \text{ Pa}]
Q2. For a soap bubble of radius [2.5 mm], find the excess pressure if [T = 4.0 × 10⁻² N/m].
[p] [= \dfrac{4T}{r}] [= \dfrac{4 × 4.0 × 10^{-2}}{2.5 × 10^{-3}}] [= 64 \text{ Pa}]
Q3. A spherical raindrop has radius [1 mm]. What happens to excess pressure if its radius doubles?
Since [p ∝ \dfrac{1}{r}], the new pressure becomes half.
Q4. Compare excess pressures of two drops with radii 1 mm and 2 mm.
[\dfrac{p_1}{p_2}] [= \dfrac{r_2}{r_1}] [= \dfrac{2}{1} = 2]
So, smaller drop has twice the excess pressure.
Q5. If the surface tension of a liquid is 0.05 N/m and the radius of the drop is 2 mm, find excess pressure.
[p] [= \dfrac{2T}{r}] [= \dfrac{2 × 0.05}{2 × 10^{-3}}] [= 50 \text{ Pa}]