Physics and Mathematics
Excess Pressure Inside a Soap Bubble
1. Statement of the Concept
A soap bubble consists of a thin film of liquid enclosing air. Since it has two surfaces — an inner and an outer — both surfaces contribute to the surface tension.
As a result, the pressure inside the bubble is greater than the pressure outside, and this pressure difference is known as the excess pressure inside the soap bubble.
2. Explanation and Mathematical Derivation
Consider a soap bubble of radius [r] and surface tension [T].
Let the pressure inside be greater than the pressure outside by [p].
When the radius increases by a small amount [dr]:
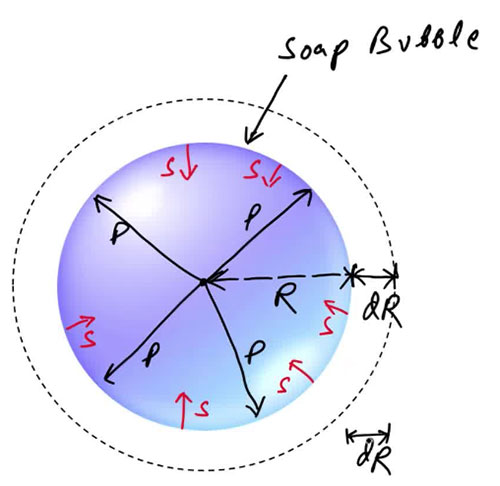
- Work done by excess pressure:
[
W = p × 4πr^2 × dr
]
- Increase in surface energy:
A soap bubble has two surfaces, so the increase in surface area is:
[
ΔA = 8πr × dr
]
Therefore,
[\text{Increase in surface energy}] [= 2T × 4πr × dr] [= 8πT r × dr]
At equilibrium,
[\text{Work done by pressure}] [= \text{Increase in surface energy}]
[
p × 4πr^2 × dr = 8πT r × dr
]
Simplifying,
[p = \dfrac{4T}{r}]
Hence,
Excess pressure inside a soap bubble: [\boxed {p = \dfrac{4T}{r}}]
3. Dimensions and Units
- Formula: [p = \dfrac{4T}{r}]
- Dimensions of T: [M¹L⁰T⁻²]
- Dimensions of p: [ML⁻¹T⁻²]
- SI Unit: Pascal (Pa)
- CGS Unit: dyne/cm²
4. Key Features
- Soap bubble has two surfaces, both experiencing surface tension.
- Excess pressure is twice that of a liquid drop of the same radius.
- The smaller the bubble, the greater the excess pressure.
- Explains why smaller bubbles collapse and merge with larger ones.
- The internal pressure is always greater than the external pressure by [p = \dfrac{4T}{r}].
5. Important Formulas to Remember
| System | Excess Pressure (p) | Remarks |
|---|---|---|
| Liquid Drop | [p = \dfrac{2T}{r}] | One surface |
| Soap Bubble | [p = \dfrac{4T}{r}] | Two surfaces |
| Liquid Jet | [p = \dfrac{T}{r}] | One curvature |
6. Conceptual Questions with Solutions
1. Why is excess pressure in a soap bubble twice that in a liquid drop?
Because a soap bubble has two surfaces (inner and outer), both contributing surface tension effects.
2. What is the physical reason for higher pressure inside a soap bubble?
The surface tension pulls inward on both surfaces, requiring higher internal pressure to maintain equilibrium.
3. What happens to excess pressure if the bubble’s radius is doubled?
Since [p ∝ \dfrac{1}{r}], excess pressure becomes half.
4. How does excess pressure change if surface tension is increased?
It increases proportionally as [p ∝ T].
5. Why do small bubbles collapse into larger bubbles?
Small bubbles have higher excess pressure, forcing air into larger ones until equilibrium is reached.
6. Does gravity affect the excess pressure?
No, excess pressure depends only on surface tension and radius, not on gravity.
7. Why does a soap bubble form a sphere?
A sphere provides the minimum surface area for a given volume, minimizing surface energy.
8. Can a soap bubble exist in a vacuum?
No, it collapses because no external air pressure exists to balance the internal pressure.
9. What is the direction of the excess pressure?
It acts from inside outward, balancing the inward pull of surface tension.
10. How does temperature affect the excess pressure?
Increasing temperature decreases surface tension, reducing excess pressure.
11. What happens when a soap bubble rises in air?
External pressure decreases, causing the bubble to expand and possibly burst.
12. Why do bubbles eventually burst?
Evaporation and thinning of the film reduce strength, making the pressure difference unsustainable.
13. How can a bubble be stabilized?
Adding glycerin or sugar reduces evaporation and increases film strength.
14. Is the inside air denser than outside?
Yes, due to the higher internal pressure.
15. How does excess pressure explain bubble merging?
The high-pressure small bubble forces air into larger bubbles, equalizing pressures.
7. FAQ / Common Misconceptions
1. Is excess pressure same as atmospheric pressure?
No, it is the **difference** between internal and external pressures.
2. Does excess pressure depend on air inside the bubble?
No, only on surface tension and bubble radius.
3. Are both surfaces of a soap bubble identical?
Nearly yes — both contribute equally to surface tension effects.
4. Does excess pressure exist in a solid sphere?
No, only liquids or thin films with surface tension experience it.
5. Why doesn’t a bubble exist indefinitely?
Because the liquid film gradually thins and bursts due to evaporation and drainage.
6. Is excess pressure affected by external pressure changes?
The absolute internal pressure changes, but the **difference** (excess pressure) remains governed by [T] and [r].
7. Does excess pressure change with height in air?
Only slightly, due to small variations in external air pressure.
8. Can the bubble’s internal pressure be equal to outside pressure?
No, equilibrium requires internal pressure to exceed external pressure.
9. Does excess pressure depend on film thickness?
Not directly; it depends mainly on radius and surface tension.
10. Why is the soap film colorful?
Due to interference of light in the thin film — unrelated to excess pressure.
8. Practice Questions (with Step-by-Step Solutions)
Q1. Find the excess pressure inside a soap bubble of radius [2 × 10⁻³ m]. Surface tension [T = 4.0 × 10⁻² N/m].
Solution:
[p] [= \dfrac{4T}{r}] [= \dfrac{4 × 4.0 × 10^{-2}}{2 × 10^{-3}}] [= 80 \text{ Pa}]
Q2. Compare the excess pressure inside a soap bubble and a liquid drop of the same radius.
[\dfrac{p_{\text{bubble}}}{p_{\text{drop}}}] [= \dfrac{4T/r}{2T/r}] [= 2]
So, the soap bubble has twice the excess pressure.
Q3. Calculate the radius of a soap bubble if the excess pressure is [50 Pa] and surface tension [T = 5 × 10⁻² N/m].
[r] [= \dfrac{4T}{p}] [= \dfrac{4 × 5 × 10^{-2}}{50}] [= 4 × 10^{-3} \text{ m}] [= 4 \text{ mm}]
Q4. If the surface tension of a soap solution decreases by 20%, how does excess pressure change?
Since [p ∝ T], it decreases by 20%.
Q5. Two bubbles of radii 2 mm and 4 mm are connected. Which way will air flow?
Smaller bubble has higher excess pressure → air flows from smaller to larger bubble until pressures equalize.