Physics and Mathematics
Rise of Liquid in a Capillary Tube (Ascent Formula)
1. Concept Overview
Capillarity (or capillary action) is the phenomenon of rise or fall of a liquid in a narrow tube (called a capillary tube) when it is dipped into the liquid.
This happens due to the combined effects of surface tension and adhesive/cohesive forces between the liquid and the tube material.
2. Explanation and Mathematical Derivation
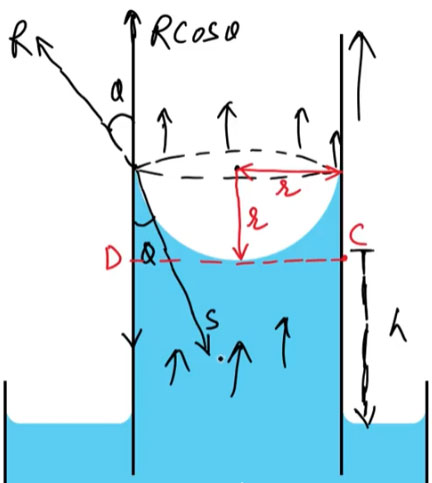
When a capillary tube of radius [r] is dipped in a liquid:
- If adhesive forces > cohesive forces, the liquid rises in the tube (e.g., water in glass).
- If cohesive forces > adhesive forces, the liquid falls (e.g., mercury in glass).
Let the surface tension of the liquid be [T] and the angle of contact between the liquid and tube be [θ].
The vertical component of surface tension force along the inner surface of the tube supports the weight of the liquid column.
Balance of Forces
[
2πrT \cosθ = πr^2 h ρ g
]
Where:
- [h] = height (or depression) of the liquid column
- [ρ] = density of liquid
- [g] = acceleration due to gravity
- S = [T] Surface Tension
Expression for Capillary Rise or Fall
[h = \dfrac{2T \cosθ}{r ρ g}]
This shows that the height of rise (or depression) is:
- Directly proportional to [T] and [cosθ]
- Inversely proportional to [r] and [ρ]
3. Dimensions and Units
| Quantity | Symbol | Dimensions | SI Unit |
|---|---|---|---|
| Surface Tension | [T] | [M T⁻²] | N/m |
| Radius of Tube | [r] | [L] | m |
| Density | [ρ] | [M L⁻³] | kg/m³ |
| Height | [h] | [L] | m |
4. Key Features
- The smaller the radius, the greater the rise of the liquid.
- Capillarity depends on the nature of the liquid and material of the tube.
- For a wetting liquid, [θ < 90°], so [cosθ] is positive → rise occurs.
- For a non-wetting liquid, [θ > 90°], so [cosθ] is negative → depression occurs.
- Capillary rise is independent of the length of the tube, provided it is sufficient to allow the rise.
5. Important Formulas to Remember
| Concept | Formula |
|---|---|
| Capillary Rise | [h = \dfrac{2T \cosθ}{r ρ g}] |
| Weight of Liquid Column | [W = πr^2 h ρ g] |
| Upward Force due to Surface Tension | [F = 2πrT \cosθ] |
6. Conceptual Questions with Solutions
1. Why does water rise in a glass capillary tube?
Because adhesive forces between water and glass are stronger than cohesive forces among water molecules, making θ acute, so cosθ is positive and rise occurs.
2. Why does mercury fall in a glass tube?
Because cohesive forces in mercury dominate adhesive forces with glass, θ > 90°, making cosθ negative, causing depression.
3. What happens to capillary rise if the tube radius is doubled?
As h ∝ 1/r, doubling the radius reduces the rise to half.
4. Why is there no capillary action in very wide tubes?
Because surface tension effects are negligible when the radius is large.
5. What causes a concave meniscus?
Adhesive forces > cohesive forces, so the liquid wets the surface, producing a concave meniscus (e.g., water in glass).
6. What causes a convex meniscus?
Cohesive forces > adhesive forces, so the liquid does not wet the surface (e.g., mercury in glass).
7. How does gravity affect capillary rise?
It opposes the rise. Greater gravity (g) results in smaller h as h ∝ 1/g.
8. What is the effect of temperature on capillary rise?
As temperature increases, surface tension decreases, so capillary rise decreases.
9. What happens to capillary rise in a liquid of higher density?
Since h ∝ 1/ρ, rise decreases with increasing density.
10. Why is kerosene sometimes used in thermometers instead of mercury?
Kerosene wets glass (acute θ), providing easier movement in narrow tubes.
11. Why is capillary action important in plants?
It helps transport water upward through narrow xylem tubes.
12. Does surface tension alone cause capillarity?
No, it acts along with adhesive and cohesive forces to produce the effect.
13. Why is the capillary rise higher in thin tubes?
Because the ratio of surface area to volume is greater for thinner tubes, producing more effect of surface tension per unit weight.
14. Why does the meniscus in mercury appear higher at the center?
Because mercury does not wet the glass, producing a convex surface that curves upward in the middle.
15. If two capillary tubes of different radii are dipped in the same liquid, what happens?
The smaller tube will show greater rise because h ∝ 1/r.
7. FAQ / Common Misconceptions
1. Capillarity happens only in water.
False. It occurs in any liquid with surface tension and adhesive/cohesive interaction (e.g., mercury shows depression).
2. Capillary rise is due to suction inside the tube.
False. It is due to surface tension and intermolecular forces, not air pressure difference.
3. Larger tubes show more capillary rise.
False. Smaller tubes show greater rise as h ∝ 1/r.
4. Surface tension and adhesion are independent.
False. Adhesion affects the direction of surface tension at the contact line, determining θ.
5. Capillary rise is independent of temperature.
False. It decreases as temperature increases (since T decreases).
6. Capillary rise depends on the length of the tube.
False. It depends only on r, T, θ, ρ, and g.
7. Meniscus shape has no physical meaning.
False. It reflects the balance of adhesive and cohesive forces.
8. Capillary rise is same for all liquids in same tube.
False. It varies with surface tension, contact angle, and density.
9. Capillary action violates gravity.
False. It counteracts gravity due to molecular forces but still obeys physical laws.
10. Capillarity cannot occur in space.
False. It can, and is actually enhanced due to negligible gravity.
8. Practice Questions (with Step-by-Step Solutions)
Q1. A capillary tube of radius [0.5 mm] is dipped in water ([T = 0.075 N/m], [ρ = 1000 kg/m³], [θ = 0°], [g = 9.8 m/s²]). Find the rise of water.
Solution:
[h] [= \dfrac{2T \cosθ}{r ρ g}] [= \dfrac{2 × 0.075 × 1}{0.5×10^{-3} × 1000 × 9.8}] [= 0.0306 \ m] [= 3.06 \ cm]
Q2. For mercury ([T = 0.465 N/m], [ρ = 13600 kg/m³], [θ = 135°], [r = 0.5 mm]), find the depression.
Solution:
[h] [= \dfrac{2T \cosθ}{r ρ g}] [= \dfrac{2 × 0.465 × (-0.707)}{0.5×10^{-3} × 13600 × 9.8}] [= -0.0098 \ m] [= -0.98 \ cm]
Hence, mercury depresses by 0.98 cm.
Q3. If the radius of the capillary tube is reduced to half, what happens to the height of the liquid column?
Solution:
[h ∝ 1/r]. Hence, halving r doubles the height h.
Q4. Calculate the capillary rise of water if the temperature increases such that surface tension reduces by 10%.
Solution:
[h ∝ T], so a 10% decrease in T leads to a 10% decrease in h.
Q5. Explain why kerosene rises higher than water in the same capillary tube.
Solution:
Kerosene has lower density and acute angle of contact, leading to greater rise as [h ∝ (cosθ)/ρ].