Physics and Mathematics
Atomic or Molecular Theory of Magnetism
1. Statement of the Concept / Concept Overview
The Atomic or Molecular Theory of Magnetism explains magnetism based on the microscopic behavior of atoms and their electrons.
According to this theory:
- Every atom behaves like a tiny magnet due to the motion of electrons.
- Magnetism in materials arises because of:
(a) Orbital motion of electrons
(b) Spin motion of electrons - These motions create magnetic dipole moments.
- In ordinary materials, the magnetic moments of individual atoms are randomly oriented, so net magnetism is zero.
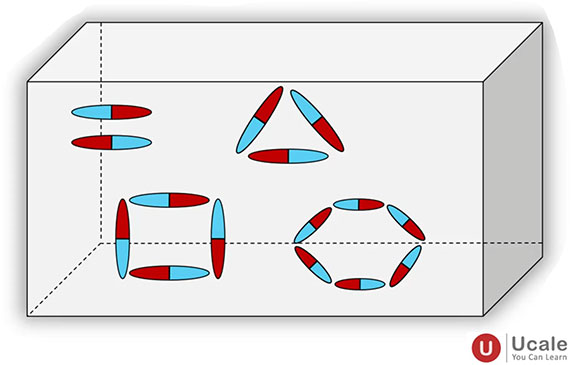
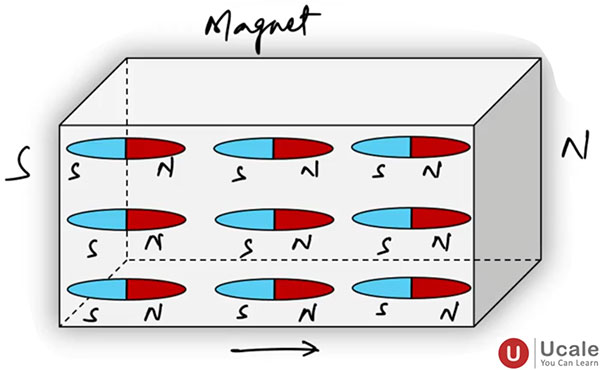
Image Credit: Ucale.org - In magnetic materials (like iron, cobalt, nickel), groups of atoms have their magnetic moments aligned in small regions called domains, giving rise to observable magnetism.
This theory is the foundation of understanding the different types of materials — paramagnetic, diamagnetic, ferromagnetic, etc.
2. Clear Explanation and Mathematical Derivation
(A) Origin of Magnetic Moment in an Atom
Electrons contribute to the magnetic moment due to:
1. Orbital Magnetic Moment
An electron revolving in an orbit of radius [r] with speed [v] constitutes a current:
[I = \dfrac{e}{T} = ev / (2\pi r)]
The magnetic moment is:
[\mu_{orbital} = I \cdot A] [= \dfrac{ev}{2\pi r} \cdot \pi r^2] [= \dfrac{evr}{2}]
2. Spin Magnetic Moment
Electrons also spin about their axes, contributing a magnetic moment:
[\mu_{spin}] [= g_s \mu_B \sqrt{s(s+1)}]
where:
- [\mu_B] = Bohr magneton [= 9.27 \times 10^{-24} A m^2]
- [g_s] = spin gyromagnetic ratio (= 2 approximately)
Total Magnetic Moment of an Atom
The resultant atomic magnetic moment is:
[\vec{\mu}{atom}] [= \vec{\mu}{orbital} + \vec{\mu}_{s pin}]
3. Dimensions and Units
| Quantity | Symbol | Dimensions | SI Unit |
|---|---|---|---|
| Magnetic moment | [\mu] | [M L^2 T^{-2} A^{-1}] | ampere–square meter (A·m²) |
| Bohr magneton | [\mu_B] | same as above | A·m² |
4. Key Features
- Every electron has intrinsic spin and behaves like a tiny magnet.
- Orbital motion also produces a magnetic moment.
- In most materials, magnetic moments cancel because they are randomly oriented.
- In ferromagnetic materials, atoms align to form magnetic domains.
- Magnetization occurs when domains align under an external magnetic field.
- Heating or hammering a magnet disturbs domain alignment and reduces magnetism.
- Above a certain temperature (Curie temperature), materials lose ferromagnetism.
5. Important Formulas to Remember
| Concept | Formula |
|---|---|
| Orbital magnetic moment | [\mu_{orbital} = \dfrac{evr}{2}] |
| Bohr magneton | [\mu_B = \dfrac{e\hbar}{2m_e}] |
| Total atomic magnetic moment | [\mu_{atom} = \mu_{orbital} + \mu_{spin}] |
| Magnetization | [M = \dfrac{\text{net magnetic moment}}{\text{volume}}] |
6. Conceptual Questions with Solutions
1. Why does every atom behave like a tiny magnet?
Because electrons possess orbital and spin motions, both of which generate magnetic dipole moments, so each atom behaves like a small magnet.
2. Why do most materials show no magnetism?
Because atomic magnetic moments are randomly oriented and cancel out, leading to zero net magnetic moment.
3. What are magnetic domains?
Small regions inside a ferromagnetic material where a large number of atomic magnetic moments are aligned in the same direction.
4. Why do ferromagnets show strong magnetism?
Because domains can grow and align with each other when an external magnetic field is applied, creating strong net magnetization.
5. Why does heating reduce magnetism?
Heat causes increased atomic vibrations, which disturb domain alignment and reduce magnetization.
6. What happens at Curie temperature?
The material loses ferromagnetism because thermal agitation completely destroys domain alignment.
7. Why do diamagnetic materials repel magnetic fields?
Induced magnetic moments develop opposite to the applied magnetic field due to electron orbital changes, creating a weak repulsion.
8. Why are Bohr magnetons important?
They represent the smallest fundamental magnetic moment contributed by an electron and act as a unit for atomic magnetic moments.
9. How do unpaired electrons affect magnetism?
Unpaired electrons contribute net magnetic moment; more unpaired electrons generally produce stronger magnetism.
10. Why is iron more magnetic than copper?
Iron has unpaired electrons that form domains, while copper has fully filled orbitals and no significant net magnetic moment.
11. Why does hammering a magnet reduce its magnetism?
Mechanical vibrations disturb the alignment of domains, reducing net magnetization.
12. Why does placing a magnet in a magnetic field strengthen it?
The external field helps align its domains more uniformly, increasing its magnetization.
13. Why are paramagnetic substances weakly attracted?
Paramagnets have some unpaired electrons but no domain structure, so their alignment is temporary and weak.
14. Why are ferromagnetics used to make permanent magnets?
They hold aligned domains even after the external magnetic field is removed due to strong interatomic interactions.
15. Why does magnetism originate primarily from electrons and not protons?
Because electrons have much lower mass, much higher magnetic moment, and dominate atomic magnetic behavior.
7. FAQ / Common Misconceptions
1. Do atoms physically act like tiny bar magnets?
No. The comparison is conceptual; atoms create magnetic fields due to electron motion, not due to small physical magnets.
2. Do all unpaired electrons guarantee strong magnetism?
No. Ferromagnetism requires domain formation and cooperative alignment, not just unpaired electrons.
3. Does a magnet lose magnetism instantly when heated?
No. Magnetization gradually decreases and becomes zero at the Curie temperature.
4. Are magnetic domains visible?
No. They are microscopic regions and can only be studied with special experimental methods.
5. Do diamagnetic materials have no electrons?
They do have electrons; their filled orbitals cause the induced magnetic moment to oppose the external field.
6. Is magnetism caused by the nucleus?
No. Electron motion (orbital + spin) is the main source of atomic magnetism.
7. Is magnetic field inside a domain zero?
No. Inside a domain, magnetic moments are well aligned, creating a strong local magnetic field.
8. Are ferromagnets always magnetic?
No. A ferromagnetic material in its unmagnetized state has randomly oriented domains and shows no net magnetism.
9. Is magnetization equal to magnetic moment?
No. Magnetization is the magnetic moment per unit volume.
10. Does breaking a magnet destroy its poles?
No. Each piece forms its own N and S poles because domains realign within each piece.