Physics and Mathematics
Charle’s Law
1. Statement of the Law
Charles’s Law states that:
“The volume of a given mass of gas is directly proportional to its absolute temperature, if the pressure remains constant.”
Mathematically,
[ V \propto T] [\quad] [\text{(at constant pressure)} ]
or
[ \dfrac{V}{T}] [= \text{constant} ]
2. Explanation and Mathematical Derivation
Let a given mass of gas have volume [V_1] at temperature [T_1], and [V_2] at temperature [T_2], both measured at constant pressure.
According to Charles’s Law:
[\dfrac{V_1}{T_1}] [= \dfrac{V_2}{T_2}]
or
[\dfrac{V_2}{V_1}] [= \dfrac{T_2}{T_1}]
This shows that when the temperature of a gas increases (at constant pressure), its volume also increases in the same ratio.
3. Dimensions and Units
- Volume (V): [L³] (SI unit: m³)
- Temperature (T): [K] (Kelvin is the absolute temperature scale)
4. Key Features
- Pressure remains constant.
- Temperature must always be measured in Kelvin for direct proportionality.
- Graph between Volume and Temperature is a straight line passing through the origin when plotted in Kelvin scale.
- At [T = 0,K], the extrapolated volume of gas becomes zero (conceptual — gases actually liquefy before reaching this point).
5. Important Formulas to Remember
| Formula | Meaning |
|---|---|
| [ V \propto T ] | Basic relation at constant pressure |
| [ \dfrac{V_1}{T_1}] [= \dfrac{V_2}{T_2} ] | Comparative form for two states |
| [ V] [= V_0 (1 + \alpha T) ] | Linear expansion form, where [\alpha] is coefficient of expansion |
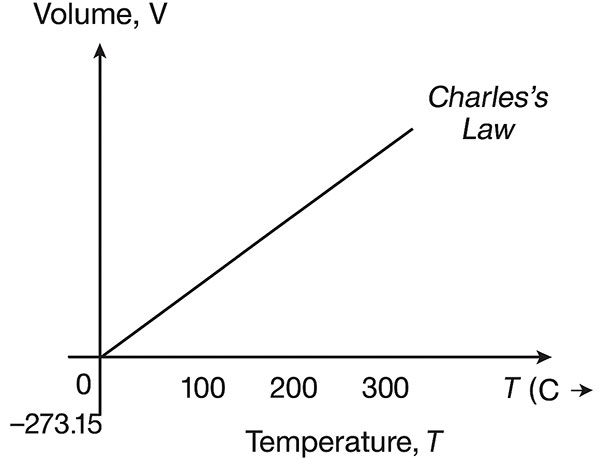
6. Graphical Understanding
(a) Volume–Temperature (V–T) Graph
A straight line passing through the origin when temperature is measured in Kelvin.
When temperature is in Celsius, the line cuts the temperature axis at [-273.15°C], representing absolute zero.
(b) Graphical Illustration
(See image below for visual understanding of Charles’s Law)
7. Conceptual Questions with Solutions
1. What remains constant in Charles’s Law?
Pressure remains constant while studying the variation of volume with temperature.
2. What happens to the volume of a gas if temperature is doubled at constant pressure?
Since \[V \propto T\], doubling the temperature (in Kelvin) doubles the volume.
3. Why must temperature be measured in Kelvin in Charles’s Law?
Because the Kelvin scale starts from absolute zero, ensuring direct proportionality between volume and temperature.
4. What does the intercept at –273.15°C represent?
It represents **absolute zero**, where theoretically, molecular motion ceases.
5. Can volume become zero in practice?
No, before reaching absolute zero, gases liquefy, so zero volume is not physically possible.
6. How does Charles’s Law explain hot air balloons?
Heating the air inside increases its temperature and volume, reducing density, making the balloon rise.
7. What is the significance of absolute zero in this law?
It is the theoretical temperature at which the volume of gas becomes zero.
8. If the volume of gas is 200 cm³ at 27°C, what will it be at 127°C?
[ \dfrac{V_1}{T_1}] [= \dfrac{V_2}{T_2}] [\Rightarrow \dfrac{200}{300}] [= \dfrac{V_2}{400}] [\Rightarrow V_2] [= 266.7\text{cm}^3 ]
9. Is Charles’s Law valid for all temperatures?
Only for gases that behave ideally; at very low temperatures, deviations occur.
10. Which graph best shows Charles’s Law?
A straight line between **V and T (Kelvin)**.
8. FAQ / Common Misconceptions
1. Is pressure constant in Charles’s Law?
Yes, the law applies only when pressure remains constant.
2. Does Charles’s Law apply to solids and liquids?
No, it applies only to gases.
3. Why is Celsius scale not used directly in calculations?
Because zero on the Celsius scale does not represent zero molecular motion.
4. Can gases actually reach 0 K?
No, 0 K is unattainable experimentally; it is only a theoretical concept.
5. What happens when a gas is cooled at constant pressure?
Its volume decreases in direct proportion to the drop in temperature.
6. Why do car tires deflate in winter?
Low temperature reduces air volume inside, hence pressure decreases.
7. What is the slope of V–T graph?
It is the **coefficient of thermal expansion of gas**.
8. Does this law explain expansion of gases?
Yes, Charles’s Law quantitatively explains the thermal expansion of gases.
9. What is the temperature corresponding to zero volume?
\[-273.15°C\], i.e., absolute zero.
10. What is the relationship between Charles’s Law and Absolute Temperature Scale?
The Kelvin scale was developed based on Charles’s Law, ensuring volume ∝ temperature.
9. Practice Questions (with Step-by-Step Solutions)
Q1. A gas occupies 500 mL at 27°C. What will be its volume at 127°C, pressure remaining constant?
Solution:
[\dfrac{V_1}{T_1}] [= \dfrac{V_2}{T_2}]
[\dfrac{500}{300}] [= \dfrac{V_2}{400}] [\Rightarrow V_2] [= 666.7 \text{mL}]
Q2. The volume of a gas is 400 cm³ at 20°C. At what temperature will the volume be 600 cm³?
[\dfrac{400}{293}] [= \dfrac{600}{T_2}] [\Rightarrow T_2] [= 439.5K] [= 166.5°C]
Q3. Why does a football appear deflated in cold weather?
Because as temperature decreases, volume of air decreases at constant pressure, reducing internal pressure.
Q4. A gas at 0°C occupies 2 L. What will be its volume at 273°C at constant pressure?
[\dfrac{2}{273}] [= \dfrac{V_2}{546}] [\Rightarrow V_2] [= 4\text{L}]
Q5. Explain how Charles’s Law helps in hot air balloon design.
The heated air expands (increase in volume), reducing its density, allowing it to lift the balloon upwards.