Physics and Mathematics
Coulomb’s Law
1. Statement of the Law / Concept Overview
Coulomb’s Law states that:
The electrostatic force between two stationary point charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. The force acts along the line joining the charges.
Mathematically,
[F = k \dfrac{q_1 q_2}{r^2}]
Where:
- [F] = Electrostatic force
- [q_1, q_2] = Charges
- [r] = Separation between charges
- [k = \dfrac{1}{4\pi\varepsilon_0}] [= 9\times10^9\ \text{N m}^2\text{C}^{-2}]
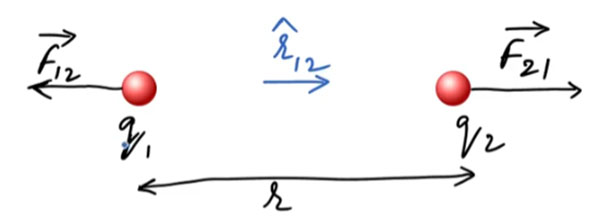
2. Clear Explanation and Mathematical Derivation
Direct Proportionality with Charges:
[F \propto q_1 q_2]
Greater charge → stronger force.
Inverse Proportionality with Distance:
[F \propto \dfrac{1}{r^2}]
This inverse-square nature resembles the law for gravitation—but with charge instead of mass.
Combining Both:
[F \propto \dfrac{q_1 q_2}{r^2}]
Introducing the constant [k = \dfrac{1}{4\pi\varepsilon_0}]:
[F = k \dfrac{q_1 q_2}{r^2}]
Direction of Force:
- If [q_1 q_2 > 0] → Like charges → repulsion
- If [q_1 q_2 < 0] → Unlike charges → attraction
Vector Form:
If [\hat{r}] is the unit direction vector from [q_1] to [q_2], then:
[\vec{F} = k\dfrac{q_1 q_2}{r^2}\hat{r}]
3. Dimensions and Units
| Quantity | Dimensions | SI Unit |
|---|---|---|
| Force [F] | ([MLT^{-2}]) | Newton (N) |
| Charge [q] | ([IT]) | Coulomb (C) |
| Coulomb constant [k] | ([M L^3 T^{-4} I^{-2}]) | N·m²·C⁻² |
4. Key Features
- Valid only for point charges or spherical charge distributions.
- Electrostatic force is central and conservative.
- Follows the inverse square law like gravity.
- Much stronger than gravitational force between elementary particles.
- Superposition holds: combined forces are vector sums.
- Independent of medium if performed in vacuum.
- In a medium with dielectric constant [\varepsilon],
[
[F = \dfrac{1}{4\pi\varepsilon} \dfrac{q_1 q_2}{r^2}]
]
5. Important Formulas to Remember
| Concept | Formula |
|---|---|
| Coulomb’s Law (scalar) | [F] [= k\dfrac{q_1 q_2}{r^2}] |
| Coulomb’s constant | [k] [= \dfrac{1}{4\pi\varepsilon_0}] |
| Vector form | [\vec{F}] [= k\dfrac{q_1 q_2}{r^2}\hat{r}] |
| In a medium | [F] [= \dfrac{1}{4\pi\varepsilon}\dfrac{q_1 q_2}{r^2}] |
| Relative permittivity effect | [F_{\text{medium}}] [= \dfrac{F_{\text{vacuum}}}{\varepsilon_r}] |
| Force ratio for gravity comparison | [\dfrac{F_e}{F_g}] [= \dfrac{k q_1 q_2}{G m_1 m_2}] |
6. Conceptual Questions with Solutions (15)
1. Why does Coulomb’s law use inverse square dependence?
Because electric field spreads uniformly in 3D space, and flux conservation implies intensity decreases as \[1/r^2\]. Hence force also varies as \[1/r^2\].
2. Why is Coulomb’s law valid only for point charges?
Because extended bodies create non-uniform charge distributions. Point charges or spherical symmetry ensure that the law applies accurately.
3. What happens to the force if the distance between charges is doubled?
Force becomes: \[ [F’ = \dfrac{F}{2^2} = \dfrac{F}{4}] \]
4. Why does force increase in a medium with low permittivity?
Because \[F \propto \dfrac{1}{\varepsilon_r}\]. Lower \(\varepsilon_r\) → greater force.
5. Does Coulomb’s law apply to moving charges?
No. Moving charges produce magnetic fields; full electromagnetic force (Lorentz force) is required.
6. If one of the charges is doubled, how does force change?
Force also doubles because \[ [F \propto q_1 q_2] \]
7. Why do like charges repel?
Due to the nature of the electric field configuration around similar charges, the field lines diverge, creating a repulsive interaction.
8. Why is Coulomb’s constant so large?
Because the permittivity of free space \[\varepsilon_0\] is extremely small; hence \[ [k = \dfrac{1}{4\pi\varepsilon_0}] \] is very large.
9. How does force behave if both charges change sign?
Force direction reverses (repulsion ↔ attraction), but magnitude remains same because \[|q_1 q_2|\] unchanged.
10. What happens if one charge is zero?
No force exists: \[F = 0\]. Charge interacts only if both charges are non-zero.
11. Why is vacuum used as reference medium?
Because vacuum has the lowest possible permittivity, allowing it to serve as a universal standard.
12. Is Coulomb’s force conservative?
Yes. Work done is path-independent because electric field is conservative for static charges.
13. Can Coulomb force exist inside a conductor?
No, because electric field inside a conductor is zero in electrostatic condition.
14. Why can’t Coulomb’s law be applied at atomic distances?
Because quantum effects dominate; classical electrostatics no longer applies.
15. Why do forces form an action-reaction pair?
Newton’s Third Law holds. Force exerted by \[q_1\] on \[q_2\] equals and opposes that by \[q_2\] on \[q_1\].
7. FAQ / Common Misconceptions (10)
1. Misconception: Coulomb’s force is always repulsive.
Incorrect. It is repulsive for like charges and attractive for unlike charges.
2. Misconception: Force depends only on magnitude of charges.
No. It also depends on distance and medium.
3. Misconception: Coulomb’s law works for any two objects.
No. Only valid for point charges or spherical distributions.
4. Misconception: Zero net charge means zero force.
Each charged particle still interacts individually; net charge does not determine pairwise force.
5. Misconception: If charges are doubled, force quadruples.
No. Force doubles if one charge is doubled; quadruples only if **both** are doubled.
6. Misconception: Coulomb’s law doesn’t depend on medium.
It does. Force reduces by factor \[\varepsilon_r\].
7. Misconception: Direction of force depends on magnitude.
No. Direction depends only on signs.
8. Misconception: Coulomb’s law is vectorless.
No. Force is a vector; law has a full vector form.
9. Misconception: Inverse-square laws apply only to gravity.
No. Many laws (electric, light intensity, radiation) are inverse-square.
10. Misconception: Charges must touch to interact.
Incorrect. Coulomb force acts even at very large separations.
8. Practice Questions (With Step-by-Step Solutions)
Q1. Two charges [q_1 = 3\ \text{C}] and [q_2 = 6\ \text{C}] are separated by 2 m. Find force.
Solution:
[F = k\dfrac{q_1 q_2}{r^2}]
[F = 9\times10^9 \dfrac{(3)(6)}{(2)^2}]
[F = 9\times10^9 \dfrac{18}{4}]
[F = 4.05\times10^{10}\ \text{N}]
Q2. Two charges 10 cm apart attract each other with 5 N. If distance becomes 20 cm, what is the new force?
[F’ = F \left(\dfrac{r}{r’}\right)^2]
[F’ = 5 \left(\dfrac{0.1}{0.2}\right)^2] [= 5 \times \dfrac{1}{4}] [= 1.25\ \text{N}]
Q3. Charges [+4\ \text{C}] and [-2\ \text{C}] are 3 m apart. Find force magnitude.
[F = 9\times10^9 \dfrac{(4)(2)}{9}]
[F = 8\times10^9\ \text{N}]
Direction: Attraction.
Q4. Two identical charges produce a repulsive force of 16 N at 1 m. What will be force at 4 m?
[F’ = 16 \left(\dfrac{1}{4}\right)^2] [= 16 \times \dfrac{1}{16}] [= 1\ \text{N}]
Q5. In a medium with [\varepsilon_r = 5], two charges exert 20 N force in vacuum. What is force in medium?
[F_{\text{medium}}] [= \dfrac{F_{\text{vacuum}}}{\varepsilon_r}]
[F_{\text{medium}}] [= \dfrac{20}{5}] [= 4\ \text{N}]