Physics and Mathematics
Gay Lussac’s Law or Regnaults Law
1. Statement of the Law
Gay-Lussac’s Law (also called Regnault’s Law) states that:
“The pressure of a given mass of a gas is directly proportional to its absolute temperature, provided the volume remains constant.”
Mathematically,
[P \propto T] [\quad] [\text{(at constant volume)}]
or
[\dfrac{P}{T}] = [\text{constant}]
2. Explanation and Mathematical Derivation
Consider a fixed mass of gas enclosed in a container of constant volume.
Let its pressure be [P_1] at temperature [T_1], and [P_2] at temperature [T_2].
According to Gay-Lussac’s Law,
[\dfrac{P_1}{T_1} = \dfrac{P_2}{T_2}]
or
[\dfrac{P_2}{P_1} = \dfrac{T_2}{T_1}]
This means — when the temperature of a gas increases (in Kelvin), its pressure also increases proportionally, if the volume is kept constant.
3. Dimensions and Units
- Pressure (P): [M L⁻¹ T⁻²] (SI unit: Pascal or N/m²)
- Temperature (T): [K]
4. Key Features
- Volume of the gas remains constant.
- Temperature is measured on the Kelvin scale.
- The graph between pressure (P) and temperature (T) is a straight line when temperature is in Kelvin.
- Extrapolated line cuts the temperature axis at –273.15°C, indicating absolute zero pressure.
5. Important Formulas to Remember
| Formula | Description |
|---|---|
| [ P \propto T ] | Basic relation at constant volume |
| [ \dfrac{P_1}{T_1}] [= \dfrac{P_2}{T_2} ] | Comparative form |
| [ P] [= P_0(1 + \alpha T) ] | Linear relation, where [\alpha] = coefficient of pressure expansion |
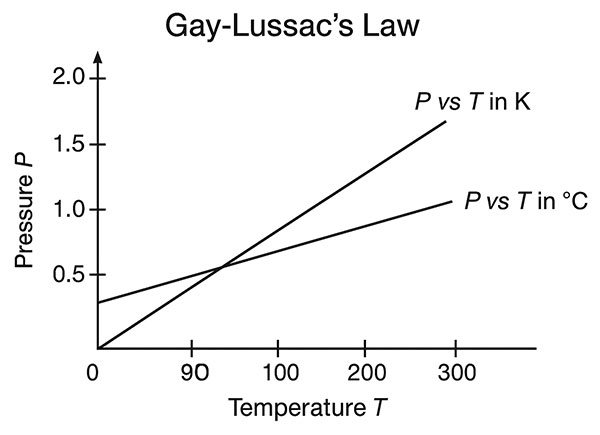
6. Graphical Understanding
(a) P–T Graph (Pressure vs Temperature)
- A straight line through the origin when T is in Kelvin.
- When temperature is in Celsius, the line cuts the temperature axis at –273.15°C.
(b) Graphical Illustration
(See the rendered diagram below for clear visualization of Gay-Lussac’s Law.)
7. Conceptual Questions with Solutions
1. What remains constant in Gay-Lussac’s Law?
The **volume** of the gas remains constant while studying the variation of pressure with temperature.
2. What is the relationship between pressure and temperature in this law?
\[ P \propto T \] (when volume is constant).
3. Why is Kelvin scale used in this law?
Because it starts from absolute zero, ensuring a true proportional relationship between P and T.
4. What happens to gas pressure if temperature doubles?
At constant volume, pressure also doubles.
5. What does the intercept at –273.15°C indicate?
It represents **absolute zero**, the temperature where gas pressure theoretically becomes zero.
6. What happens to pressure when temperature decreases?
Pressure decreases proportionally at constant volume.
7. What is the coefficient of pressure increase?
It is the fractional increase in pressure per degree rise in temperature at constant volume.
8. Why do pressure cookers cook food faster?
Because at higher temperatures, pressure increases (as per Gay-Lussac’s Law), raising boiling points.
9. What will happen if temperature of gas is reduced to 0 K?
Theoretically, pressure becomes zero, but practically gases liquefy before that.
10. How does this law relate to molecular motion?
As temperature increases, molecular kinetic energy increases, leading to higher pressure.
8. FAQ / Common Misconceptions
1. Is volume constant in Gay-Lussac’s Law?
Yes, volume remains constant while studying pressure–temperature relationships.
2. Can this law be applied to solids or liquids?
No, it applies only to gases.
3. Why is Celsius not used directly in calculations?
Because the Celsius scale does not start from zero molecular energy.
4. What happens at constant temperature?
Pressure remains constant — this is **Boyle’s Law**, not Gay-Lussac’s.
5. Is pressure directly proportional to temperature at all conditions?
Only when the gas behaves ideally and volume is constant.
6. What does absolute zero mean in this law?
It’s the temperature at which pressure theoretically becomes zero.
7. Why does a car tire burst in hot weather?
Because the air inside expands, increasing pressure as per Gay-Lussac’s Law.
8. Is Regnault’s Law different from Gay-Lussac’s Law?
No, both describe the same pressure–temperature relationship.
9. Can gases reach zero pressure?
No, gases condense to liquids before that point.
10. What is the slope of P–T graph?
The slope represents the **rate of change of pressure with temperature**.
9. Practice Questions (with Step-by-Step Solutions)
Q1. A gas has pressure 1.5 × 10⁵ Pa at 27°C. Find its pressure at 127°C if volume remains constant.
Solution:
[\dfrac{P_1}{T_1} = \dfrac{P_2}{T_2}]
[\dfrac{1.5 \times 10^5}{300}] [= \dfrac{P_2}{400}] [\Rightarrow P_2] [= 2.0 \times 10^5\text{Pa}]
Q2. The pressure of gas is 100 kPa at 0°C. At what temperature will the pressure be 150 kPa?
[\dfrac{100}{273}] [= \dfrac{150}{T_2}] [\Rightarrow T_2] [= 409.5K = 136.5°C]
Q3. Explain why aerosol cans are dangerous near heat.
When temperature increases, internal gas pressure rises rapidly, which can cause bursting.
Q4. A gas at 27°C has a pressure of 10⁵ Pa. At what temperature will its pressure be 2 × 10⁵ Pa?
[\dfrac{10^5}{300}] [= \dfrac{2 \times 10^5}{T_2}] [\Rightarrow T_2] [= 600K] [= 327°C]
Q5. What physical principle supports the working of a pressure cooker?
At higher temperature, pressure rises according to Gay-Lussac’s Law, increasing the boiling point of water and cooking food faster.