Physics and Mathematics
Kinetic Energy Per Molecule of Gas
1. Statement of the Concept
The kinetic energy of a gas molecule is the energy possessed by it due to its motion.
According to the kinetic theory of gases, the pressure of a gas is directly related to the average kinetic energy of its molecules.
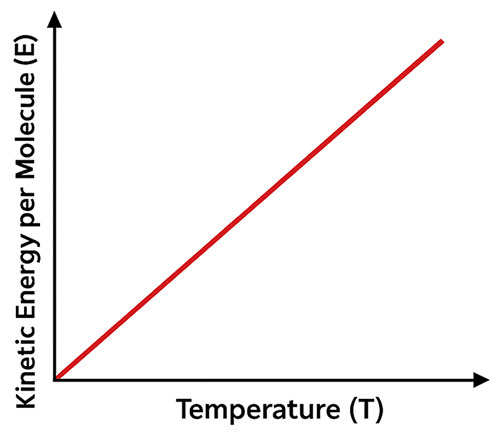
For an ideal gas, the mean kinetic energy per molecule is directly proportional to its absolute temperature.
[
E = \dfrac{3}{2} k T
]
where
- [E] → average kinetic energy per molecule
- [k] → Boltzmann constant ([1.38 \times 10^{-23} , J/K])
- [T] → absolute temperature in Kelvin
2. Clear Explanation and Mathematical Derivation
From the kinetic theory of gases, we know that
[
pV = \dfrac{1}{3} N m \overline{v^2}
]
Dividing both sides by [N]:
[
\dfrac{pV}{N} = \dfrac{1}{3} m \overline{v^2}
]
Now, from the perfect gas equation,
[
pV = N k T
]
Substituting this in the above equation:
[
kT = \dfrac{1}{3} m \overline{v^2}
]
Rearranging,
[
\dfrac{1}{2} m \overline{v^2} = \dfrac{3}{2} kT
]
But [\dfrac{1}{2} m \overline{v^2}] represents the average kinetic energy per molecule, hence
[
E = \dfrac{3}{2} kT
]
3. Dimensions and Units
| Quantity | Symbol | Dimensions | SI Unit |
|---|---|---|---|
| Kinetic Energy per molecule | [E] | [M L^{2} T^{-2}] | Joule (J) |
| Boltzmann constant | [k] | [M L^{2} T^{-2} K^{-1}] | J/K |
| Temperature | [T] | [K] | Kelvin |
4. Key Features
- The average kinetic energy per molecule depends only on temperature, not on pressure or volume.
- When temperature increases, molecular kinetic energy also increases.
- At absolute zero temperature (0 K), the average kinetic energy becomes zero.
- The relation [E = \dfrac{3}{2} kT] connects microscopic properties (energy per molecule) with macroscopic quantities (temperature).
- For one mole of gas, since [N = N_A]:
[
E_{\text{mole}} = \dfrac{3}{2} R T
]
5. Important Formulas to Remember
| Formula | Description |
|---|---|
| [E = \dfrac{3}{2} kT] | Average kinetic energy per molecule |
| [E_{\text{mole}} = \dfrac{3}{2} RT] | Kinetic energy per mole of gas |
| [\dfrac{1}{2} m \overline{v^2} = \dfrac{3}{2} kT] | Relation between velocity and temperature |
| [R = N_A k] | Gas constant relation |
| [\overline{v} = \sqrt{\dfrac{3kT}{m}}] | RMS velocity in terms of temperature |
6. Conceptual Questions with Solutions
1. On what factor does the kinetic energy of gas molecules depend?
The average kinetic energy of gas molecules depends **only on temperature**, not on the nature of the gas.
2. What happens to the kinetic energy if temperature doubles?
Since [E \propto T], doubling temperature doubles the average kinetic energy per molecule.
3. What is the kinetic energy of a molecule at absolute zero?
At [T = 0 \, K], [E = 0]. The molecules theoretically come to rest.
4. Why is kinetic energy independent of pressure?
Because pressure may change due to density variations, but energy depends solely on **temperature**, not volume or pressure.
5. How is kinetic energy related to molecular velocity?
It is given by [E = \dfrac{1}{2} m \overline{v^2}], connecting microscopic speed to temperature.
7. FAQ / Common Misconceptions
1. Do heavier gas molecules have more kinetic energy at the same temperature?
No, all gases at the same temperature have the **same average kinetic energy per molecule**, irrespective of mass.
2. Does higher pressure mean higher kinetic energy?
Not necessarily — kinetic energy depends only on **temperature**, not directly on pressure.
3. Can temperature be zero?
In theory, **absolute zero (0 K)** is the lowest possible temperature, where molecular motion stops.
4. Is kinetic energy always constant for all molecules?
No, individual molecules have different energies; only the **average kinetic energy** is fixed for a given temperature.
5. Why does heating increase pressure in a closed container?
Because increased kinetic energy causes faster collisions, raising pressure.
8. Practice Questions (with Step-by-Step Solutions)
Q1. Find the average kinetic energy of one molecule of gas at [T = 300 , K].
[E] [= \dfrac{3}{2} kT] [= \dfrac{3}{2} (1.38 \times 10^{-23}) (300)] [= 6.21 \times 10^{-21} \, J]
Q2. Calculate the kinetic energy per mole of gas at [T = 300\, K].
[E_{\text{mole}}] [= \dfrac{3}{2} RT] [= \dfrac{3}{2} (8.31)(300)] [= 3739.5\, J/mol]
Q3. At what temperature will the average kinetic energy of a gas molecule be [4.14 \times 10^{-21}\, J]?
[T = \dfrac{2E}{3k}] [= \dfrac{2 \times 4.14 \times 10^{-21}}{3 \times 1.38 \times 10^{-23}}] [= 200 \, K]
Q4. If the RMS velocity of a gas molecule doubles, what happens to its kinetic energy?
[E \propto \overline{v^2}] [\Rightarrow E \text{ becomes four times.}]
Q5. Find the average kinetic energy of one molecule of gas at 0°C.
[T = 273 \,K], [\quad] [E = \dfrac{3}{2} kT] [= \dfrac{3}{2} (1.38 \times 10^{-23}) (273)] [= 5.65 \times 10^{-21} \, J]