Physics and Mathematics
Molecular Theory of Surface Tension
1. Concept Overview
The molecular theory of surface tension explains the phenomenon of surface tension based on the cohesive forces acting between molecules of a liquid.
Every liquid molecule attracts its neighboring molecules through cohesive forces (intermolecular forces). The difference in molecular environment for molecules at the surface and those deep inside the liquid gives rise to surface tension.
2. Explanation and Derivation
(a) Molecules Inside the Liquid
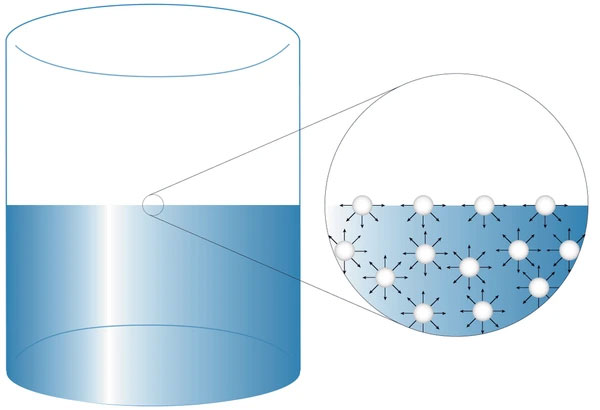
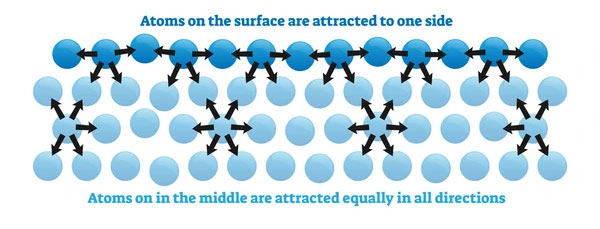
A molecule deep inside the liquid is surrounded by neighboring molecules in all directions.
Hence, the resultant cohesive force on it is zero, as all molecular attractions balance each other.
(b) Molecules at the Surface
A molecule at the surface experiences:
- Downward cohesive forces due to the molecules below.
- No upward molecular attraction, as there are no liquid molecules above the surface.
Thus, surface molecules experience a net downward force, pulling them inward.
To bring a molecule from the interior to the surface, work must be done against this force, increasing the potential energy of surface molecules.
This results in the surface having extra energy compared to the bulk — known as surface energy.
(c) Surface Tension Definition
The liquid surface behaves like a stretched elastic sheet trying to minimize its area due to molecular attraction.
If a force [F] acts along a line of length [L] on the liquid surface, then:
[
T = \dfrac{F}{L}
]
where:
- [T] → Surface tension
- [F] → Force acting tangentially
- [L] → Length along which the force acts
The SI unit of surface tension is N/m.
3. Dimensions and Units
[T] = [M^1 L^0 T^{-2}]
- SI Unit: N/m
- CGS Unit: dyne/cm
[
1 \text{N/m} = 10^3 \text{dyne/cm}
]
4. Key Features
- Surface tension arises due to cohesive molecular forces.
- It acts along the surface and is perpendicular to the line drawn in the surface.
- Liquids minimize their surface area to reduce surface energy.
- Surface tension decreases with an increase in temperature.
- Impurities (like soap) reduce surface tension.
5. Important Formulas to Remember
| Formula | Description |
|---|---|
| [T = \dfrac{F}{L}] | Definition of surface tension |
| [W = T \times \Delta A] | Work done to increase surface area |
| [E = T] | Surface energy per unit area |
| [T ∝ (1 – \dfrac{t}{t_c})] | Temperature dependence of surface tension, where [t_c] is critical temperature |
6. Conceptual Questions with Solutions
1. Why do small liquid drops assume a spherical shape?
A sphere has the minimum surface area for a given volume. Since surface tension tends to minimize surface area, small drops become spherical.
2. Why is surface tension considered a tangential force?
Because the force due to surface tension acts **along the surface** and tangentially to any line drawn on it.
3. What is the molecular reason for the existence of surface tension?
It arises due to unbalanced cohesive forces experienced by molecules at the liquid’s surface.
4. Why does surface tension decrease with temperature?
As temperature increases, molecular kinetic energy increases, weakening cohesive forces and hence reducing surface tension.
5. What happens to surface tension when impurities are added?
Surface tension generally decreases due to disruption of cohesive molecular forces by impurities.
6. Why do camphor pieces move on water?
Camphor lowers the surface tension locally; the unbalanced forces cause motion.
7. What is the difference between surface energy and surface tension?
Surface energy is energy per unit area; surface tension is force per unit length — both numerically equal in magnitude but conceptually distinct.
8. Why does hot water clean clothes better?
Hot water has lower surface tension, allowing detergents to penetrate more easily into fabric pores.
9. Why does a liquid rise in a capillary tube?
Due to adhesive and cohesive forces acting at the liquid-air interface, explained through surface tension.
10. Can a liquid have zero surface tension?
Yes, only at the critical temperature where the distinction between liquid and vapor disappears.
11. Why do insects like pond skaters walk on water?
Their weight is balanced by the surface tension force acting on their legs, preventing them from sinking.
12. What will happen if the surface tension of water becomes zero?
The surface would lose its cohesive structure and could not support small objects or droplets.
13. What is the direction of surface tension at the contact line?
It acts tangentially to the surface and perpendicular to the line of contact.
14. Why are mercury droplets spherical?
Cohesive forces in mercury are stronger than adhesive forces with glass or air, causing them to contract into spheres.
15. What is the energy required to increase the surface area of a soap film?
Work done [W = 2T × \Delta A], since soap film has two surfaces.
7. FAQ / Common Misconceptions
1. Surface tension acts vertically upward.
Incorrect. It acts tangentially along the liquid surface.
2. Surface tension and surface energy are completely different quantities.
They have the same magnitude but represent force per unit length and energy per unit area, respectively.
3. All liquids have the same surface tension.
No, surface tension depends on molecular composition and temperature.
4. Surface tension increases with temperature.
False. It decreases as temperature increases.
5. Soap solution increases surface tension.
No, it reduces surface tension, aiding cleaning action.
6. Surface tension acts only on the top surface of a liquid.
It acts at every interface between the liquid and another medium (like air).
7. Surface tension acts only when the liquid is in motion.
It exists even when the liquid is at rest.
8. Surface tension is independent of impurities.
It is affected significantly by impurities and surfactants.
9. Surface tension can never be zero.
It becomes zero at the critical temperature.
10. The molecular theory of surface tension applies only to pure liquids.
It is general, though deviations occur in mixtures or impure systems.
8. Practice Questions (with Step-by-Step Solutions)
Q1. A force of [0.03 N] is required to move a steel wire of [3 × 10^{-2} m] length over the surface of water. Find the surface tension.
Solution:
[T = \dfrac{F}{L}] [= \dfrac{0.03}{3 \times 10^{-2}}] [= 1 \text{N/m}]
Q2. What is the work done to increase the surface area of a soap film by [40 cm²], if the surface tension is [30 × 10^{-3} N/m]?
Solution:
For a soap film, two surfaces exist.
[W] [= 2T \times \Delta A] [= 2 \times 30 \times 10^{-3} \times 40 \times 10^{-4}] [= 2.4 \times 10^{-4} \text{J}]
Q3. If the surface tension of water at [20°C] is [0.073 N/m], calculate the force acting on a ring of radius [2 cm] when it is pulled out of the water surface.
Solution:
[F = 2T \times 2\pi r] [= 4\pi rT] [= 4 \times 3.14 \times 0.02 \times 0.073] [= 0.0183 \text{N}]
Q4. A capillary tube of radius [0.5 mm] is dipped in water. If the surface tension of water is [0.072 N/m], calculate the capillary rise.
Solution:
[h] [= \dfrac{2T\cos\theta}{r\rho g}] [= \dfrac{2 \times 0.072 \times 1}{0.5 \times 10^{-3} \times 1000 \times 9.8}] [\approx 0.029 \text{m}] [= 2.9 \text{cm}]
Q5. Explain how the molecular theory justifies the existence of surface energy.
Solution:
Surface molecules have higher potential energy due to unbalanced cohesive forces; thus, additional work (energy) is required to bring them to the surface, giving rise to surface energy.